"Beware the Wolf in Sheep's Clothing: Many Companies Claim GMP Compliance Without Holding a License—Verify that Your CDMO and Formulation Provider has a suitable GMP licence on the TGA Website"
Many companies claim to follow Good Manufacturing Practice (GMP) standards or offer “GMP-like” services without holding a valid GMP license. This can pose serious risks to the safety, quality, and compliance of pharmaceutical products. Unlicensed contract development and manufacturing organizations (CDMOs) may cut corners, leading to compromised formulations, subpar testing, and regulatory non-compliance, which could ultimately harm patients and businesses alike. To safeguard your products and ensure compliance, always verify that your CDMO holds a valid GMP license.
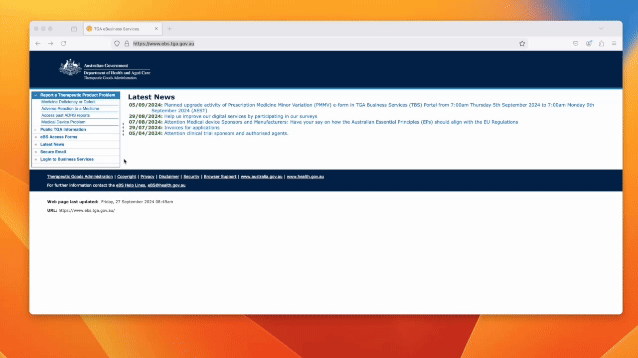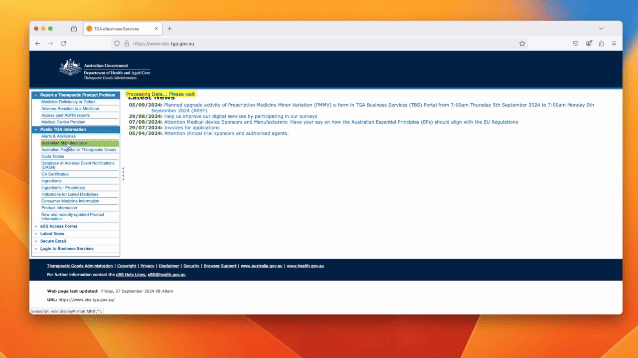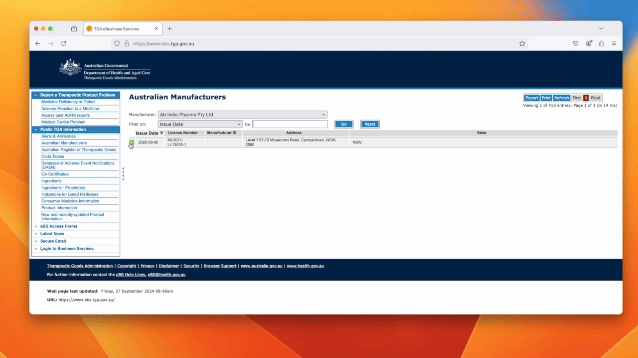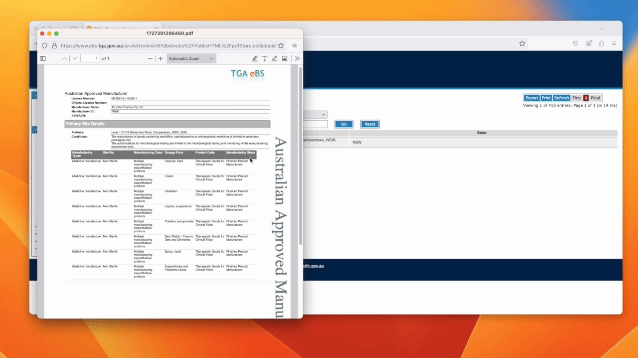
In Australia, you can check the status of a company’s GMP license through the Therapeutic Goods Administration (TGA) website by searching the publicly available TGA database for licensed manufacturers. It is as simple as following a the following simple steps….
Click on the following link and follow these 4 easy steps https://www.ebs.tga.gov.au
· Click public information in the dropdown
· Click Australian Manufacturer
· Search the manufacturer in the dropdown list
· Click on the manufactures licence
Always pay attention to the manufacturing steps and restrictions. For example, “Finished Product
Manufacture” means the CDMO can undertake all steps of manufacturing including QC, production and release for supply. Some organisations will claim GMP services but may only be able to test or store your product!
About Ab Initio - Ab Initio Pharma specializes in the GMP formulation and manufacturing of complex dosage forms, including semisolids, inhalation and nasal products, capsules and advanced lipid/nanoparticle-based systems, providing comprehensive pharmaceutical development services. Ab Initio holds GMP licence no MI-2021-LI-13035-1 for manufacture of clinical trial products including testing, blinding and release for supply. Ab Initio also holds an NSW Wholesale licence and can manufacture and supply scheduled medicines and drugs of addiction (S8) as well as testing and manufacturing S9's under permit